COVID-19 Antigen Test
Rapid Diagnostic Test for the Detection of SARS-CoV-2 Antigen
Inspire Diagnostics COVID-19 Antigen Rapid Point-of-Care Test*
The Inspire Diagnostics COVID-19 Antigen Test is a lateral flow immunochromatographic assay for the detection of extracted nucleocapsid protein antigens specific to SARS-CoV-2 in swab specimens directly collected from individuals who are suspected of COVID-19 by their healthcare providers.
As an intended point-of-care (POC)* designated test with results to be read at 10 minutes, Inspire Diagnostics COVID-19 Antigen Test allows diagnostic indication of COVID-19 by qualified healthcare professionals on a large scale.

* This test is authorized for point-of-care use by users with a CLIA certificate of wavier.
Here’s a brief video that includes a start-to-finish guide on how to conduct an Inspire Diagnostics COVID-19 antigen test.
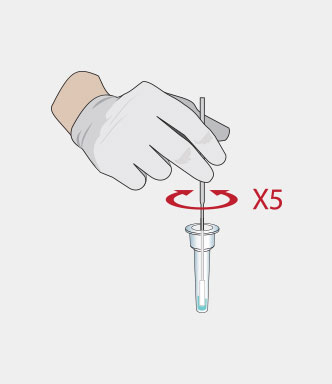
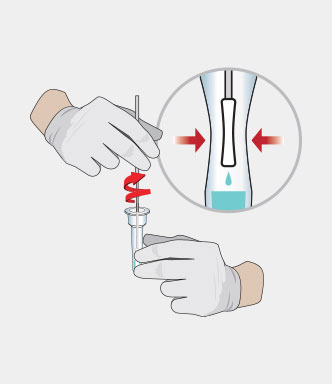
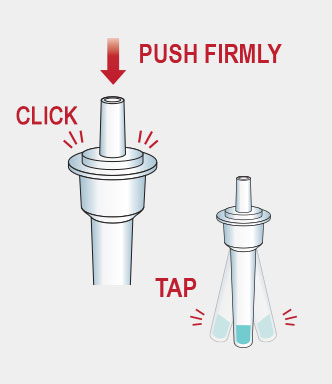
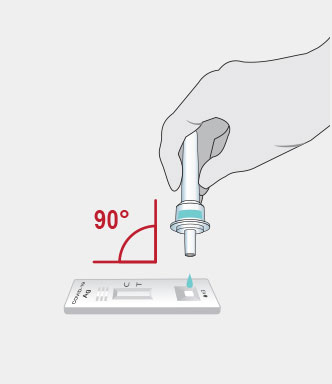
Procedure
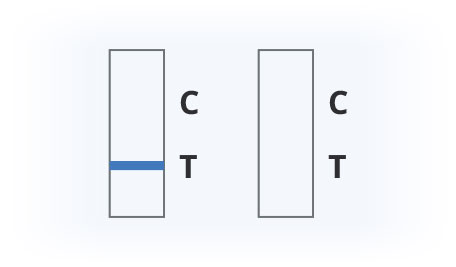
Results Interpretation
Covid-19 Positive
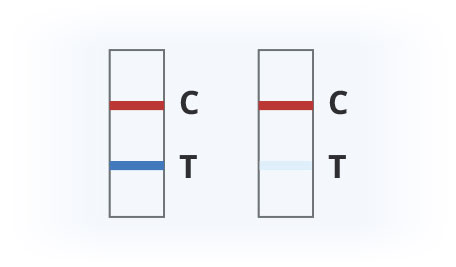
Covid-19 Negative
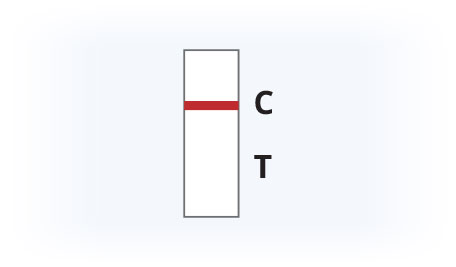
Invalid