There are a variety of tests currently available for use in the
Fight Against COVID-19
COVID-19 Testing Overview
COVID-19 Viral Test vs Antibody Test
There are a variety of tests currently available for use in the fight against COVID-19. The tests fall broadly into two categories based on their purpose. The first is a VIRAL test which tests for the presence of an active (and presumably contagious) COVID-19 infection (Active Infection Phase). Viral tests can be further classified by the target of the test. A MOLECULAR (RNA) test looks for the presence of the virus itself. An ANTIGEN test looks for the presence of certain proteins that surround the active virus (the viral envelope).
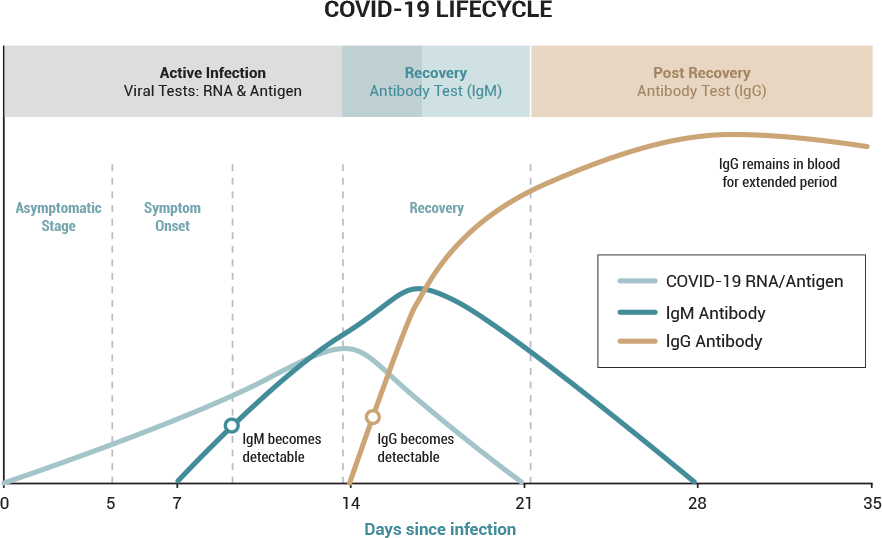
The second category of test is the ANTIBODY test. This tests for the presence of COVID-19 antibodies in the system which signifies that the person being tested has been infected by the Coronavirus and is recovering or has recovered (and likely no longer infectious). The conventional thinking is that an individual who has a certain level of antibodies in his/her system is immune for a period of time after the initial infection (although it is currently not known for how long the individual may be immune).
There are two types of antibodies that the human body produces in response to a COVID-19 infection. One is referred to as IgM and is short-lived and will disappear within days after the virus is no longer active. The second antibody is referred to as IgG. This antibody is produced after the active infection has run its course and it is present for weeks after the virus is no longer active. The level of these two antibodies can provide some indication as to which phase of the infection lifecycle the test subject is in at the time of the test: active infection phase, the recovery phase or the post-recovery phase. Some antibody tests test exclusively for the presence of IgM, some test exclusively for IgG and others test for the presence of both.
Laboratory Test vs Point-of-Care Test
Tests can also be categorized based on the setting in which the test must be conducted. Some tests are LABORATORY tests which are typically more complex with more manually-intensive processing and require that the test specimen be sent to a certified lab that has the equipment and personnel to conduct the test.
Another category of test called POINT-OF-CARE tests are designed to be, as the name implies, conducted at the point of care, be it a hospital, clinic or doctor’s office where the patient is receiving medical treatment. These tests are designed to be performed by personnel with a much lesser degree of training, typically involve much simpler specimen collection methods and few, if any, manual processing steps. Some tests are completely self-contained and do not require processing equipment to obtain a result while other point-of-care tests must be used in conjunction with test processing equipment which automates the processing of the test specimen and delivers the test result without any manual processing.
Point-of-Care Tests: Stick Tests vs Automated Analyzer Tests
Within the category of rapid point-of-care tests, there are two distinct segments. One is a stick test format for which the modern disposable pregnancy test is a prime example. These tests are simple-to-use self-contained disposable test strips which provide a qualitative test result without the need for any test processing equipment. The advantage of these types of tests is that they are typically cheaper than other test types. The primary disadvantage of this type of test is that they tend to be less accurate relative to other test types. Recently, there has been much press about a number of Chinese-made COVID-19 stick-type antibody tests that have turned out to be significantly less accurate than advertised which has cast doubt on the entire category in the eyes of many organizations. Also if testing at any significant scale, these tests are cumbersome due to the required manual entry of the test result in any central test result tracking system.
The second type of point-of-care is an automated analyzer test in which the test specimen is collected manually and then processed by a separate piece of equipment (an analyzer) in order to determine the test result. These types of tests can produce both a qualitative and semi-quantitative result depending on the methodology. These tests are typically more accurate than the stick tests. In addition, the analyzer can digitally store all test results which can then be transmitted electronically into a central repository for further analysis and monitoring.